
Energy—the ability to do work—can be divided into six different forms.

Mechanical energy is the energy associated with motion. The amount of energy depends on the mass and velocity of the relevant system or component. Objects that have greater mass or motion at a higher speed have higher kinetic (or mechanical energy). It also includes the potential for motion, such as with gravitational potential energy (for example from elevated water) or elastic potential energy (for example from a coiled spring).

Thermal energy (or heat) is a measure of kinetic energy at the molecular level. Heat and temperature are proxy measurements for the motion of molecules. One way we feel as heat is when energy transfers to our bodies from molecules colliding against us. Molecules move faster in hot weather, colliding against us with higher velocity, which feels warm. In many systems, thermal energy is held in working fluids such as steam, refrigerants, molten salts, or specialty oils. In power plants and heating districts, thermal energy is typically carried in steam. In cooling districts or refrigeration systems, thermal energy is carried in chilled water or refrigerants, such as R-142a or ammonia.

Electrical energy (or electricity) is ubiquitously familiar to the developed world as a common form of secondary energy in the built environment. This form of energy is carried in currents and driven by voltages across loads (or resistances).

Radiant energy includes those forms of energy that travel in waves. Examples include electromagnetic radiation, such as light and magnetic forces. Thus, light waves carry energy, which is a simple explanation for how sunlight can cause burns. Acoustic waves also carry energy. Microphones detect this energy to make an audio recording. Conversely, very powerful weapons destroy buildings using intense acoustic waves.

Chemical energy is the energy stored in the chemical bonds of molecules. During combustion, fuels burn and the chemical bonds break, releasing heat. The energy content of fuels depends on the composition, masses, and other properties of the molecules. Chemical energy is responsible for more than 85% of the energy consumption around the world in the form of fossil fuels (coal, petroleum, and natural gas) and bioenergy (wood, alcohols, straw, and cow dung).
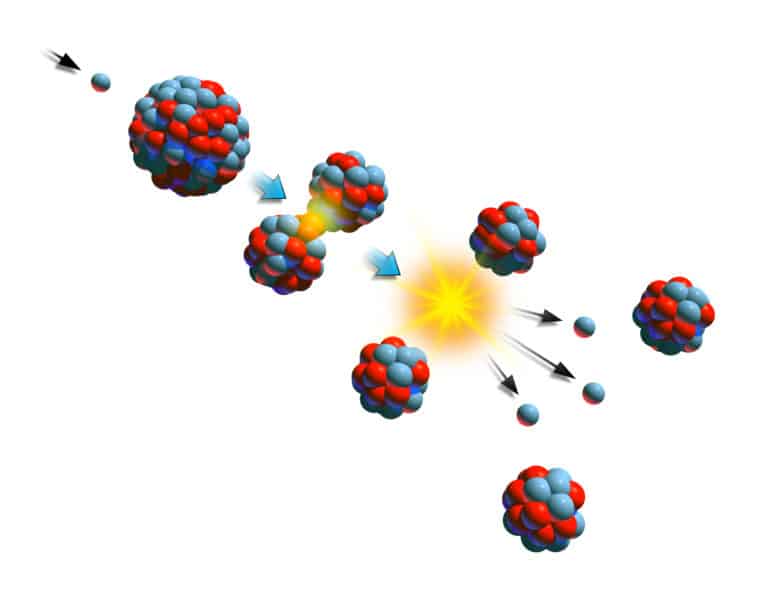
Atomic energy is the energy stored in the nucleus of atoms. During reactions, atomic bonds break, releasing heat. Einstein’s famous equation relates mass and the energy contained within: E = mc2. For this equation, E is energy, m is the mass lost during a conversion, and c is the speed of light. In other words, because the speed of light is so large (light moves at a rate of 30 billion centimeters per second), small changes in mass yield tremendous energy. During atomic reactions, minuscule amounts of mass disappear, releasing significant amounts of energy.
Image Credits: WWW.JET-PIX.DE/stock.adobe.com; Oleksandr Kostiuchenko/Shutterstock.com; seeyou/Shutterstock.com; imging/Shutterstock.com; Ufuk ZIVANA/Shutterstock.com; Peter Gudella/Shutterstock.com; Andrea Danti/Shutterstock.com.
Update your browser to view this website correctly.Update my browser now